

The cyclopentadienyl cation [C5R5]+ has been a major synthetic target for chemists for many years but has so far only been fleetingly detected, despite considerable efforts.1-8 The major driving force for this interest is that the singlet-state, five-membered ring isomer is considered to be the prototypical 4n p-electron antiaromatic compound. There has been considerable debate as to the structure of this cation and many theoretical studies have attempted to determine the minimum energy isomer.9-11 The most likely structures are a planar D5h-symmetric triplet (1) and a vinyl cyclopropenyl cation (2), although a methylene cyclobutenyl cation (3) and a nido square-based pyramidal structure (4) related to that of nido-B5H9 have also been proposed.
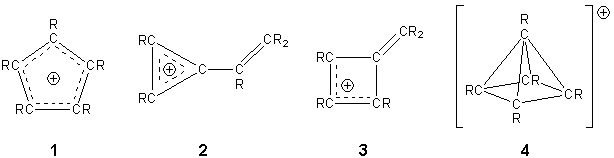
The conceptual substitution of “CR” fragments in the [C5R5]+ cation with isolobal phosphorus atoms leads to a series of inorganic analogues of this cation [C5-xR5-xPx]+ (x = 1 - 5). Recent work in this group led to the synthesis of the first phosphorus-containing analogue of the cyclopentadienyl cation, by chloride abstraction from the tricyclic compound 5. The product of this reaction was analysed by single crystal X-ray diffraction and found to have the Wadean structure nido-[3,5-tBu2-1,2,4-C2P3]+[AlCl4]- (6).12 This compound represents the first carbon/phosphorus cluster cation and the most closely related analogue of the [C5R5]+ cation yet to be synthesised and was published as a “very important paper” in Angewandte Chemie. This work was further publicised by a highlight in the same journal by Prof. Guy Bertrand.13 In solution, the cation exhibits interesting fluxional behaviour that leads to exchange of the apical and basal phosphorus atoms through a relatively low-energy (calculated) transition state, which is similar to behaviour predicted by Hoffman for nido-[C5H5]+.14
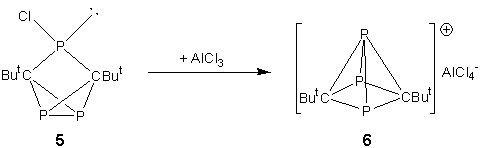
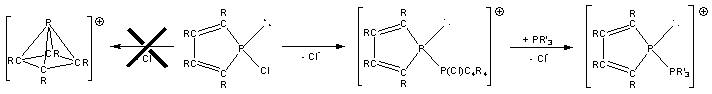
I have used a similar strategy to investigate the synthesis of the carbon rich cation [C4R4P]+ by chloride abstraction from P-chlorophospholes. Rather than forming cations with nido geometries (7), which are also predicted to be the most stable isomers in this system,10 diphosphonium cations (8) were observed. Interestingly, these compounds behave as phosphine coordinated phosphenium cations, in that they undergo rapid ligand exchange reactions with competing donors e.g. PPh3 (9). Theoretical investigations into this system suggested that the observation of phosphine stabilised [C4R4P]+ cations in this system may be due to a high-energy barrier to isomerisation from a planar to a nido structure and the high Lewis acidity of the planar-[C4R4P]+ cation.
The diphosphonium salt [Et4C4P(PPh3)][O3SCF3] (10) was synthesised by reaction of Et4C4PCl with Me3SiO3SCF3 in the presence of PPh3 and fully characterised including a structural determination by single crystal X-ray diffraction. This compound undergoes a rapid ligand exchange reaction with PPh3 in dichloromethane. The kinetics of this reaction were studied by peak shape analysis of 31P NMR spectra containing mixtures of 10 and PPh3 under different conditions using the program gNMR 4.0. The reaction was found to proceed via an SN2 mechanism with a second order rate law and a small, positive enthalpy of activation that suggests a low-energy transition state.
Group 13 Imido Clusters With High Lithium Content
The chemistry of p-block imido anions has been extensively investigated in recent years in order to better understand the isoelectronic relationship between the imido (N=R) and oxo (=O) group in main group compounds. These anions have proved to be interesting supramolecular building blocks that aggregate with metal cations to form a novel class of cluster compounds.
While the imido chemistry of group 14, 15 and 16 elements has been well studied, relatively few examples of group 13 imido compounds are known. I have successfully synthesised the following imido anions by the deprotonation of group 13 amides using alkyllithium bases.
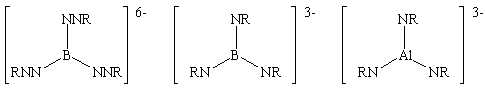
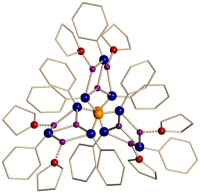
These anions aggregate with their lithium counterions to form large cage structures in the solid state that have been studied using X-ray crystallography (some examples of the structures formed by this class of compound are shown below). The planarity of the above anions complicates aggregation, as the coordination requirements of the Li+ counter-ions are not easily satisfied. This is resolved by the inclusion of other anionic fragments present in the reaction mixture and stable structures with high lithium ion content are formed. These clusters are structurally interesting and may be of use as single-source precursors to materials containing group 1, 13 and 15 elements.
 |
 |
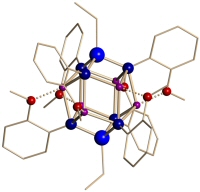 |
| A cage containing 16 Li+ cations | A cage containing 9 Li+ cations |
A dianionic cage containing [Al(R)(NR)3]4− tetraanions |
References:
1 R. Breslow, H. W. Chang, R. Hill and E. Wasserman, J. Am. Chem. Soc., 1967, 89, 1112.
2 M. Saunders, R. Berger, A. Jaffe, J. M. McBride, J. O'Neill, R. Breslow, J. M. Hoffman Jr., C. Perchonock, E. Wasserman, R. S. Hutton and V. J. Kuck, J. Am. Chem. Soc., 1973, 95, 3017.
3 P. Jützi and A. Mix, Chem. Ber., 1992, 125, 951.
4 G. A. Dushenko, I. E. Michailov, I. A. Kamenetskaya, R. V. Skachkov, A. Zhunke, K. Myugge and V. I. Minkin, Russ. J. Org. Chem., 1994, 30, 1559.
5 A. D. Allen, M. Sumonja and T. T. Tidwell, J. Am. Chem. Soc., 1997, 119, 2371.
6 T. Müller, Angew. Chem., Int. Ed. Engl., 2002, 41, 2276.
7 J. B. Lambert, Angew. Chem., Int. Ed. Engl., 2002, 41, 2278.
8 J. M. Lambert, L. Lin and V. Rassolov, Angew. Chem., Int. Ed. Engl., 2002, 41, 1429.
9 A. D. Allen and T. T. Tidwell, Chem. Rev., 2001, 101, 1333.
10 D. A. Pantazis, J. E. McGrady, J. M. Lynam, C. A. Russell and M. Green, J. Chem. Soc., Dalton Trans., 2004, 2080.
11 K. B. Wiberg, Chem. Rev., 2001, 101, 1317.
12 J. M. Lynam, M. C. Copsey, M. Green, J. C. Jeffery, J. E. McGrady, C. A. Russell, J. M. Slattery and A. C. Swain, Angew. Chem., Int. Ed. Engl., 2003, 42, 2778.
13 Y. Canac and G. Bertrand, Angew. Chem., Int. Ed. Engl., 2003, 42, 3578.
14 W. D. Stohrer and R. Hoffmann, J. Am. Chem. Soc., 1972, 94, 1661.
My Publications:
A Flexible Synthesis of Imino-l5-Stibane Dimers, M. C. Copsey, S. B. Gallon, S. K. Grocott, J. C. Jeffery, J. M. Slattery, C. A. Russell, Inorg. Chem., submitted.
The aggregation of lithium imido borates, C. A. Russell, J. M. Slattery, Phosphorus, Sulfur, Silicon Relat. Elem., 2004, 179, 931–932.
Heterobimetallic lithium alkyltriimido aluminate cages containing the [R′Al(NR)3]4- tetraanion (R′ = Bun, Et; R = 2-OMeC6H4), M. C. Copsey, J. C. Jeffery, C. A. Russell, J. M. Slattery, J. A. Straughan, Chem. Commun., 2003, 18, 2356–2357.
Selective preparation of the [3,5-tBu(2)-1,2,4-C2P3]- ion and synthesis and structure of the cationic species nido – [3,5-tBu(2)-1,2,4-C2P3]+ isoelectronic with [C5R5]+, J. M. Lynam, M. C. Copsey, M. Green, J. C. Jeffery, J. E. McGrady, C. A. Russell, J. M. Slattery, A. C. Swain, Angew. Chem. Int. Ed. Engl., 2003, 42, 2778–2782 (VIP).
A bis(imido)organoarsenate dianion incorporating n-butyllithium, M. C. Copsey, J. C. Jeffery, A. P. Leedham, C. A. Russell, J. M. Slattery, J. Chem. Soc., Dalton Trans., 2003, 11, 2103–2104.